- Accueil
- mcv4
- Sanofi Pasteur Announces FDA Approval of Menactra Meningococcal Conjugate Vaccine Indication for Infants
Sanofi Pasteur Announces FDA Approval of Menactra Meningococcal Conjugate Vaccine Indication for Infants
5 (642) · € 34.00 · En Stock
/PRNewswire/ -- Sanofi Pasteur, the vaccines division of the sanofi-aventis Group (EURONEXT: SAN and NYSE: SNY), announced today that the U.S. Food and Drug

Safety and Immunogenicity of a Meningococcal Quadrivalent Conjugate Vaccine in Five- to Eight-Year-Old Saudi Arabian Children Previously Vaccinated with Two Doses of a Meningococcal Quadrivalent Polysaccharide Vaccine

An evaluation of emerging vaccines for childhood meningococcal disease, BMC Public Health

Meningococcal vaccine - Wikipedia
Full article: Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT, Nimenrix™): A review of its immunogenicity, safety, co-administration, and antibody persistence

Meningococcal Meningitis (MenACWY) Vaccine MenQuadfi® (Meningococcal [Groups A, C, Y, W] Conjugate Vaccine)
PREGNANCY / DRUG EXPOSURE VIA PARENT DATA COLLECTION FORM
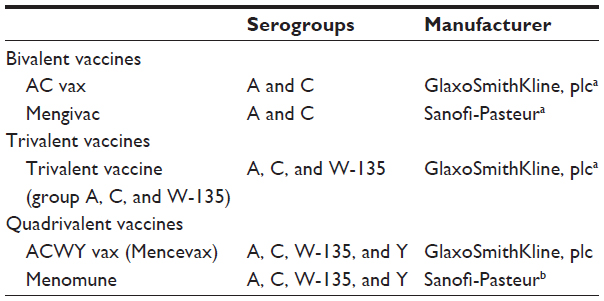
Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate v

Global Key Players in the Vaccines Market

The status of COVID-19 vaccines in India: A review

Menactra Meningococcal Vaccine, Sanofi Pasteur at Rs 3700/vial in Mumbai

Menactra Meningococcal Diphtheria Toxoid Conjugate Vaccine, Sanofi Pasteur at Rs 3600/piece in Jalandhar












