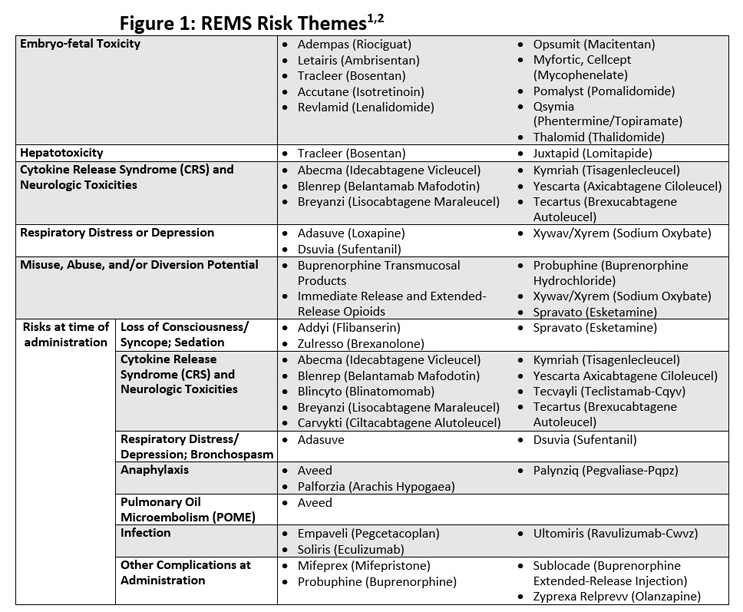
Understanding The FDA's Current Focus On Risk Evaluation And
4.8 (445) · € 22.00 · En Stock
lt;p>The FDA recently asked for comments about how the government handles vendor change requests from drug sponsors with risk evaluation and mitigation strategies. So, we asked a REMS expert to help us understand why the agency is focusing on the broad-reaching program and what it could mean for drug manufacturers with REMS products in their portfolios.</p>

The United States Food and Drug Administration (FDA) Recommends a
FDA Medical Device Classification: Classes and Examples

Understanding FDA Cleared vs Approved vs Granted for Medical

Mobile Health App Interactive Tool

Risk Management and Communication: Building Trust and Credibility
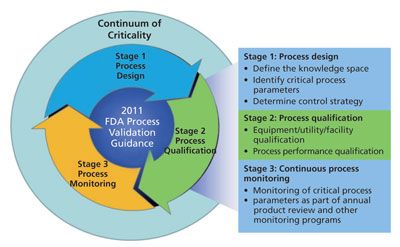
Determining Criticality-Process Parameters and Quality Attributes

5 Risk Assessment tools used by Life Sciences Companies
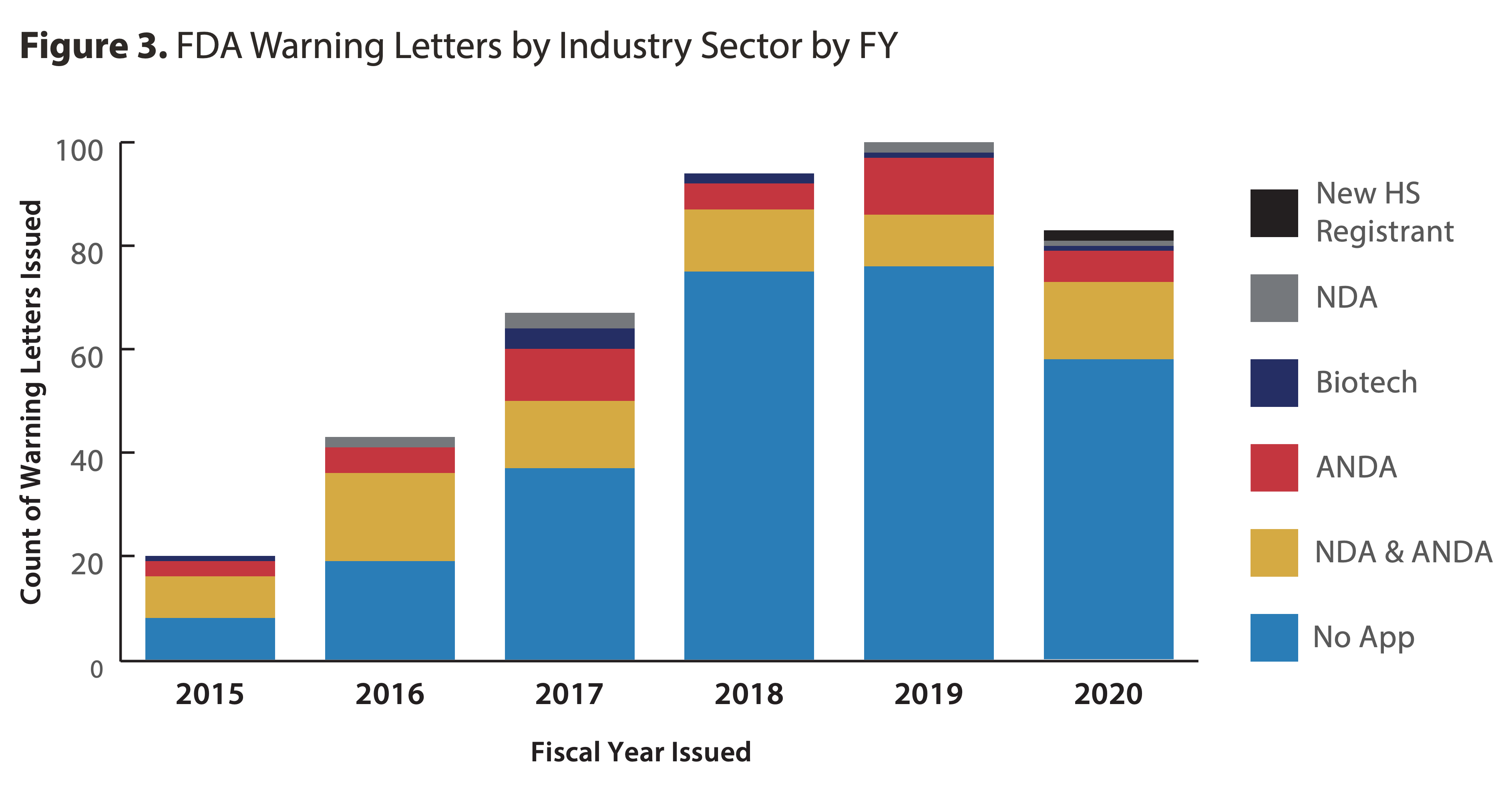
FDA Warning Letter & Inspection Observation Trends [Updated 2023]

New FDA Guidance Answers Questions On RBM Use

Understanding The FDA's Current Focus On Risk Evaluation And

Food and Drug Administration - Wikipedia
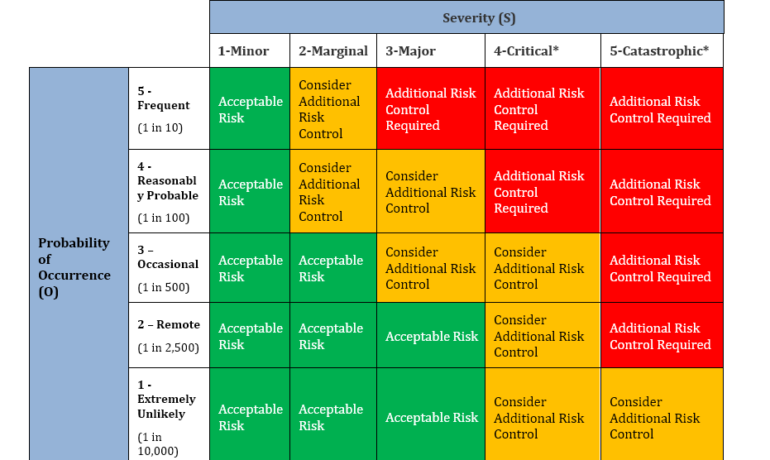
Medical Device Quality Risk Management · Qualcy eQMS
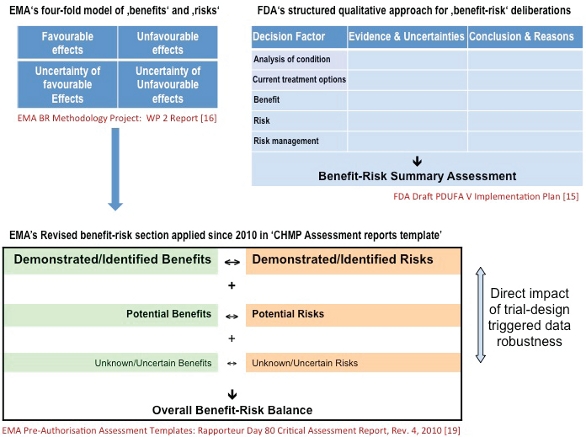
Clinical Quality Risk Management: Growing Impact for Favorable