Update on REMS-Required Testing During COVID-19 Pandemic - MPR
5 (228) · € 23.00 · En Stock
“The completion of some REMS-required laboratory testing or imaging studies may be difficult because patients suspected of having COVID-19 may be self-isolating and/or subject to quarantine,” said FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD.

COVID-19: Federal Efforts Could Be Strengthened by Timely and Concerted Actions
Medicina, Free Full-Text

Lift Unnecessary Restrictions: Access to Medication Abortion During the COVID-19 Pandemic

REMS Program & Liver Function JYNARQUE® (tolvaptan) tablets

Mailing List Readiness and Emergency Management for Schools Technical Assistance Center

Medicina, Free Full-Text
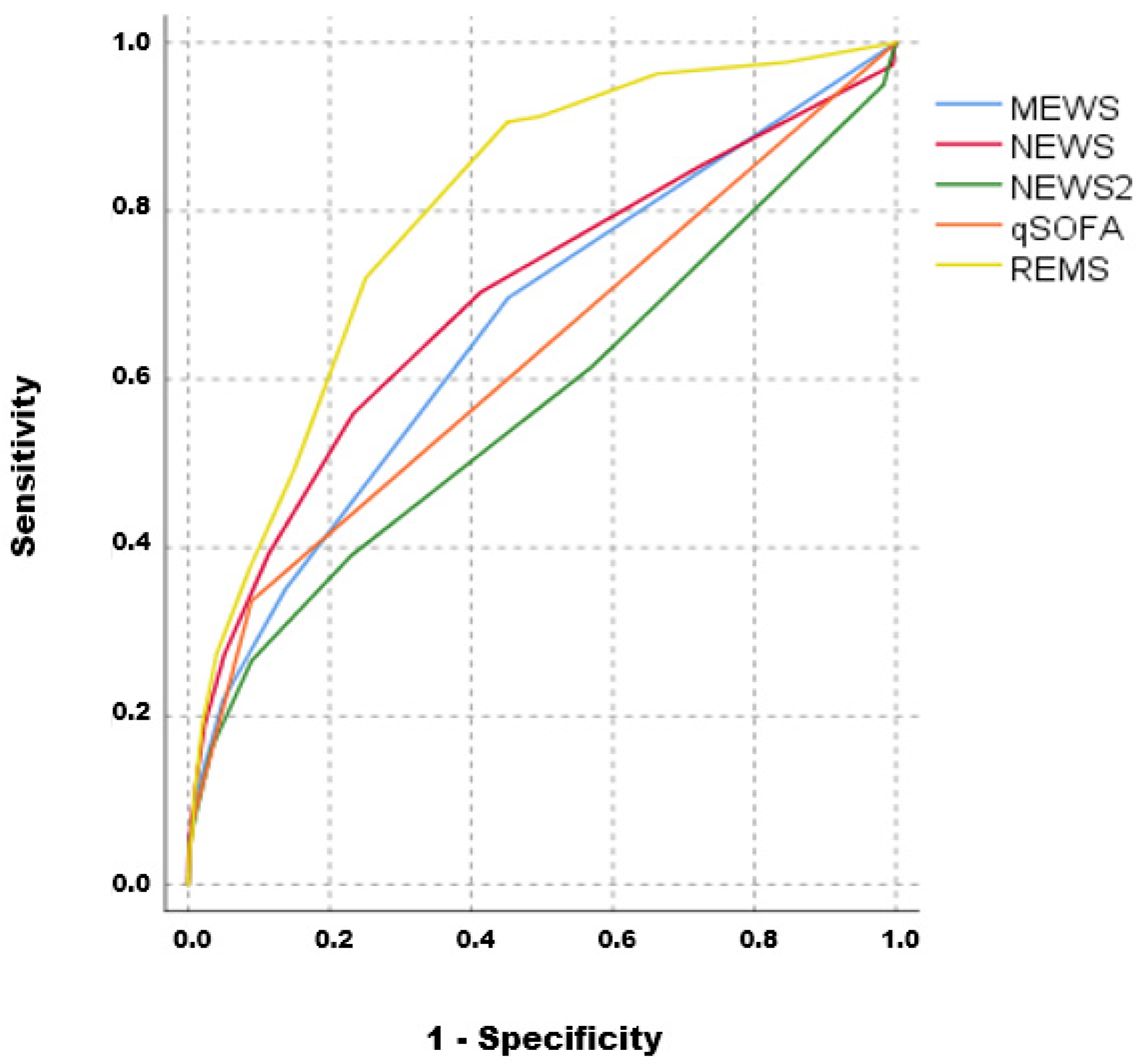
Diagnostics, Free Full-Text

Archived Webinars Readiness and Emergency Management for Schools Technical Assistance Center

The Risk Evaluation and Mitigation Strategy (REMS) Public Dashboard: Improving Transparency of Regulatory Activities

Coronavirus (COVID-19) Information
Medication Abortion Care Is Safe and Effective—It's Time Everyone Has Equal Access












